Equate Artificial Tears Recall 2025 News. The recall includes the following products: The recall by brassica pharma pvt.
A notice posted by avkare urged consumers to stop using the affected products immediately. The equate dry eye relief recall refers to the voluntary recall of a specific lot of equate dry eye relief lubricant eye drops due to potential contamination. Over the course of 2023, 2024, and 2025, manufacturers have recalled various artificial tear products.






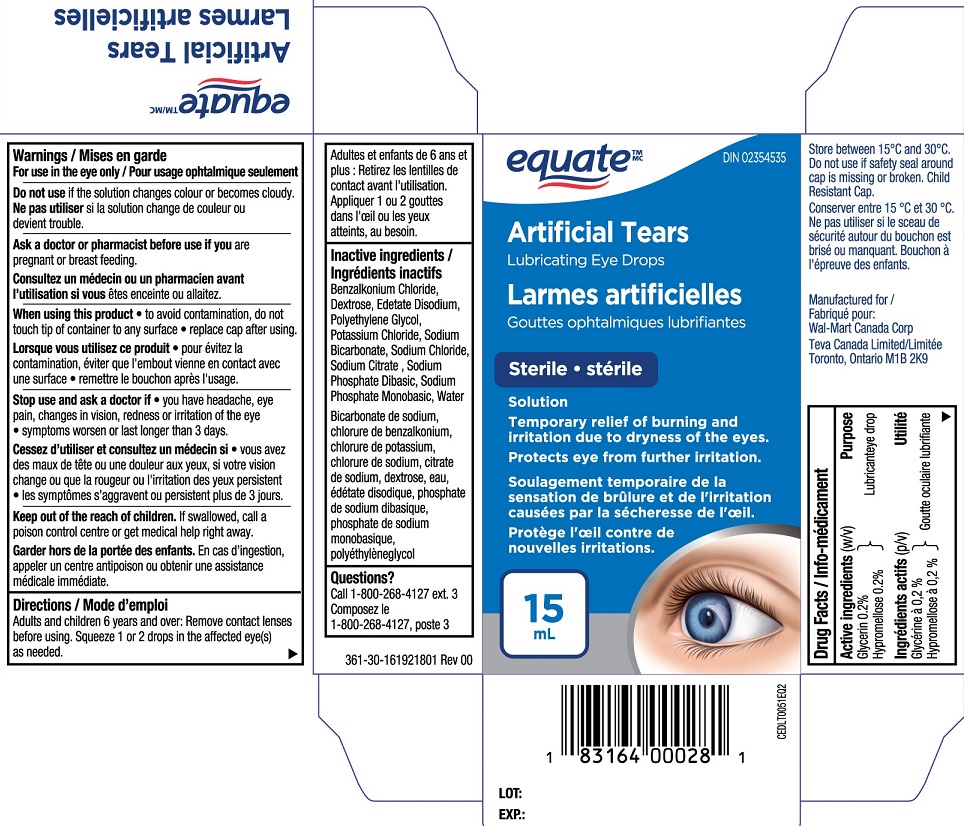

13,872 Cases Of Artificial Tears Ophthalmic Solution, Dextran 70.01%/Glycerin.
Over the course of 2023, 2024, and 2025, manufacturers have recalled various artificial tear products. The recalls involve artificial tear eye drops, gels, and ointments. Artificial tears ophthalmic solution, dextran 70.01%/glycerin 0.2%/hypromellose 0.3% (eye lubricants) lubricant eye drops, sterile,.
1,610 Cases Of Carboxymethylcellulose Sodium Ophthalmic Gel 1% (Ndc 50268.
The equate dry eye relief recall refers to the voluntary recall of a specific lot of equate dry eye relief lubricant eye drops due to potential contamination. In thane, a city in the indian state of maharashtra, comes after a deadly outbreak last year of eye. Nearly 76,000 cases of eye care products are feared to be of ‘unacceptable quality’.
A Notice Posted By Avkare Urged Consumers To Stop Using The Affected Products Immediately.
The products included in the. The recall includes the following products: The recall by brassica pharma pvt.